
Formaldehyde is used in various organic synthesis and reactions. It is used in the textile industry in the area of crosslinking molecules to vary the properties of fabrics. In the food industry it is used to reinforce and improve packaging of meats and produce. It can help improve the water resistance of biodegradable collagen films.
| Chinese name | 甲醛 | English name | Formaldehyde |
|---|---|---|---|
| Chinese alias | 蚁醛;福尔马林; | English alias | Formol;Formaldehyde;Oxymethylene;Methanal;Formic aldehyde; 查看更多英文别名 |
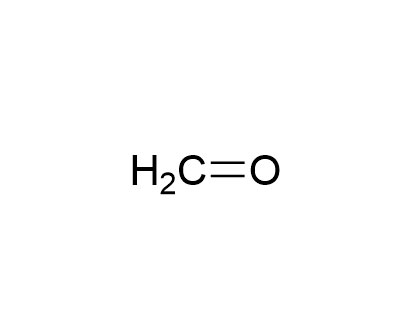
| CAS | 50-00-0 | Molecular formula | CH2O |
| Molecular weight | 30.02600 | Accurate mass | 30.01060 |
| PSA | 17.07000 | LOGP | 0.45100 |
| UNII | 1HG84L3525 |
|---|
Appearance and characteristics:
Colorless gas
Density:
0.8153 g/cm3 (−20 °C)
Boiling point:
-19 °C
Melting point:
-92 °C
Flash point:
50 ° C (37% formaldehyde aqueous solution, containing 15% methanol, closed cup method); 85 ° C (37% formaldehyde aqueous solution, without methanol, closed cup method)
Refractive index:
n20/D 1.377
Water solubility:
Easy to dissolve,>100 g/100 ml (20 ° C)
Stability:
Stable, flammable
Storage conditions:
The warehouse is ventilated, low-temperature, and dry, and stored separately from H pore forming agents, alkalis, and strong oxidants
Steam density:
White crystal
Steam pressure:
>1 atm (20 °C)
RTECS:
LP8925000
Safety instructions:
S26;S36/37/39;S45;S51;S53;S60
Hazard category code:
R23/24/25; R34; R43; R45
WGK Germany:
2
Dangerous goods transportation code:
UN 2209 8 / PGIII
Customs code:
2912110000
Hazard category:
S26;S36/37/39;S45;S51;S53;S60
Packaging grade:
8
Dangerous goods signs:
T; C
Signal words:
Danger
Danger signs:
Hazard description:
Hazard prevention instructions:
Formaldehyde is a popular chemical product with a wide range of applications, simple production processes, and sufficient raw material supply. It is the backbone of the downstream product tree of methanol, with an annual production of about 25 million tons worldwide. About 30% of methanol is used to produce formaldehyde. But formaldehyde is a low concentration aqueous solution, which is not convenient for long-distance transportation from an economic perspective, so factories are generally set up near the main consumer market, and import and export trade is also very rare. In industry, methanol oxidation method and natural gas direct oxidation method are mainly used:
1. Methanol oxidation method: At 600-700 ℃, methanol, air, and water are directly oxidized through silver catalysts or catalysts such as copper and pentoxide to produce formaldehyde. Formaldehyde is absorbed by water to obtain formaldehyde solution. The total reaction is exothermic, but 50-60% of formaldehyde is generated through oxidation reaction, while the rest is generated through hydrogen reaction. The by-products are carbon monoxide and carbon dioxide, methyl formate, and formic acid. The conversion rate of methanol is 80%, and the yield is 85% to 90% based on methanol. This method has mature technology and high yield, and is widely adopted by domestic and foreign production plants.
2. Natural gas oxidation method: At 600~680 ℃, a mixture of natural gas and air is directly oxidized to formaldehyde through oxide catalysts such as iron and molybdenum, and absorbed with water to obtain formaldehyde solution:
3. Methanol vapor is passed through a copper or silver catalyst at 300 ℃ to produce methanol dehydrogenation. Formaldehyde gas absorbs water content of 36% to 40%, which is formaldehyde solution. Distille the commercially available formaldehyde solution to remove impurities and supplement with methanol to obtain the reagent formaldehyde solution.
4. The dimethyl ether oxidation method uses dimethyl ether, a byproduct of the synthesis of methanol by high-pressure synthesis of synthesis gas, as raw material, and metal oxides as catalysts for oxygen oxidation.
5. The methanol dehydrogenation method directly dehydrogenates methanol to obtain anhydrous formaldehyde, while producing hydrogen gas as a byproduct. This process is a highly attractive method for preparing formaldehyde. The key to its progress lies in the improvement of the performance of process catalysts.
6. Mix the vaporized methanol with air and water vapor washed with alkali at a volume ratio of 1:1.8-2.0:0.8-1.0, heat to 115-120 ℃ for reaction, and control the reaction temperature at 600-650 ℃ and pressure at 0.3-0.5MPa under the action of silver catalyst. After the reaction is complete, rapidly cool the reactants to 80-85 ℃, absorb them with water, and then distill to remove unreacted methanol. The kettle solution is treated with anion exchange resin, and an appropriate amount of polymerization inhibitor is added to the formaldehyde solution. Stir and mix to obtain the finished product.
7. Gas formaldehyde automatically polymerizes into cyclic trimeric formaldehyde at room temperature.
8. Formaldehyde can easily react with ammonia or ammonium salts to form hexamethylenetetramine, also known as urotropin.
Formaldehyde is an important organic raw material, mainly used in the plastic industry (such as the production of phenolic resins, urea formaldehyde plastics - electric jade), synthetic fibers (such as the synthesis of vinylon - polyvinyl formal), leather industry, pharmaceuticals, dyes, etc. Formaldehyde has bactericidal and preservative properties and can be used to soak biological specimens. Its dilute solution (0.1-0.5%) can be used in agriculture to soak and disinfect seeds. The commonly used catalytic oxidation method in industry is to produce formaldehyde from methanol. Formaldehyde can react with silver ammonia solution to produce a silver mirror reaction, causing a thin layer of shiny metallic silver to adhere to the inner wall of the test tube (the silver in the synthesized state is reduced, and formaldehyde is oxidized); Reacting with the newly prepared suspension of copper hydroxide to form a red precipitate of cuprous oxide.