
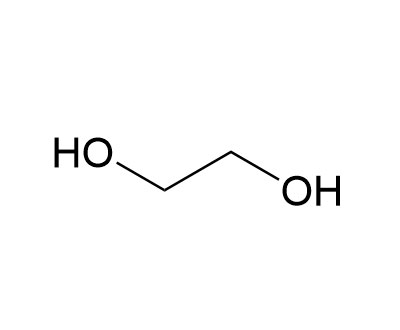
乙二醇,又名甘醇。化学式HOCH2—CH2OH。一种简单的二元醇。无色无臭、有甜味液体,能与水以任意比例混合。用作溶剂、防冻剂以及PETE等的原料。 乙二醇对动物有毒性,人类致死剂量估计为1.6 g/kg,不过成人服食30毫升已有可能引致死亡。
| Chinese name | 乙二醇 | English name | Ethylene glycol |
|---|---|---|---|
| Chinese alias | 甘醇;1,2-亚乙基二醇;乙撑二醇;次乙基甘醇; | English alias | 1,2-dihydroxy ethane;Ethane-1,2-diol;1,2-Ethanediol;1,2-Dihydroxyethane;2-hydroxy ethanol; |
| CAS | 107-21-1 | Molecular formula | C2H6O2 |
| Molecular weight | 62.06780 | Accurate mass | 62.03680 |
| PSA | 40.46000 | LOGP | -1.02900 |
| RTECS | KW2975000 |
|---|---|
| MDL | MFCD00283324 |
| EINECS | 203-473-3 |
| PubChem | 24859450 |
| BRN | 505945 |
Appearance and characteristics:
Transparent viscous liquid
Density:
1.220 g/mL at 25 °C
Boiling point:
196-198 °C(lit.)
Melting point:
-13 °C(lit.)
Freezing point:
-11.5℃
Flash point:
230 °F
Refractive index:
n20/D 1.431(lit.)
Water solubility:
miscible
Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Storage conditions:
Warehouse ventilation, low temperature drying
Steam density:
2.1 (vs air)
Steam pressure:
0.08 mm Hg ( 20 °C)
RTECS:
KW2975000
Safety instructions:
S26
Hazard category code:
R22
WGK Germany:
3
Dangerous goods transportation code:
UN 1219 3/PG 2
Customs code:
2905310000
Dangerous goods signs:
Xn
Hazard prevention instructions:
Danger signs:
Hazard description:
Signal words:
Warning
1. The chloroethanol method is obtained by hydrolyzing chloroethanol as a raw material in an alkaline medium. The reaction is carried out at 100 ℃ to first form ethylene oxide, which is then hydrolyzed under pressure at 1.01 MPa to produce ethylene glycol.2. Ethylene oxide hydration method includes direct hydration method and catalytic hydration method. The hydration process can be carried out under normal pressure or under pressure. The atmospheric pressure water method generally uses a small amount of inorganic acid as a catalyst and conducts the reaction at 50-70 ℃. Direct hydration method: Catalytic hydration method: The molar ratio of epoxyethane to water in pressurized hydration method should be higher, above 1:6, to reduce the side reaction of ether formation. The temperature of this reaction is 150 ℃ and the pressure is 147kPa. Ethylene glycol is obtained by hydration.
3. Currently, there is a gas-phase catalytic hydration method that uses silver oxide as a catalyst and aluminum oxide as a carrier to react at 150-240 ℃ to produce ethylene glycol.
4. Direct hydration method of ethylene: Ethylene is oxidized in acetic acid solution in the presence of catalysts (such as antimony oxide TeO2, palladium catalyst) to form monoacetate or diacetate, which is further hydrolyzed to obtain ethylene glycol.
5. Ethylene oxide and water undergo hydration reaction under the action of sulfuric acid catalyst, and the reaction solution is neutralized with alkali, evaporated, and distilled to obtain the finished product. Alternatively, ethylene oxide and water can be used to produce ethylene glycol at a certain temperature and pressure, while producing diethylene glycol, triethylene glycol, and polyethylene glycol as by-products. The reaction solution is evaporated, concentrated, dehydrated, and refined to obtain qualified products and by-products.
6. The reaction formula for formaldehyde method is as follows:
7. Using industrial grade ethylene glycol as raw material, after vacuum distillation, collect the middle distillate at 1333Pa.
8. Vacuum distill ethylene glycol, and dry the main fraction with anhydrous sodium sulfate for a long time. Then, use a good distillation column to vacuum distill again.
Mainly used in the manufacture of resins, plasticizers, synthetic fibers, cosmetics, and as solvents and antifreeze agents for engine formulations.